|
From rxpgnews.com Haematology
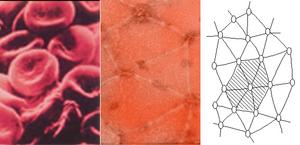
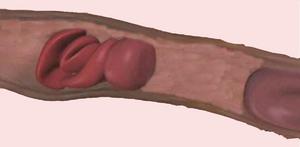
A human red blood cell is a dimpled ballerina, ceaselessly spinning, tumbling, bending, and squeezing through openings narrower than its width to dispense life-giving oxygen to every corner of the body. In a paper published in the October issue of Annals of Biomedical Engineering, which was made available online on Oct. 21, a team of UCSD researchers describe a mathematical model that explains how a mesh-like protein skeleton gives a healthy human red blood cell both its rubbery ability to stretch without breaking, and a potential mechanism to facilitate diffusion of oxygen across its membrane.
Robert Skelton, a professor of mechanical and aerospace engineering at the Jacobs School of Engineering and a co-author of the study, employed the unorthodox approach of modeling the proto-filaments as if they were part of a tensegrity structure. Artists have been more familiar with rod-and-cable tensegrity structures than scientists. Sung asked Skelton to collaborate on her red blood cell project because Skelton and his students have pioneered the development of rigorous scientific tools to analyze the movement and balance of forces in many types of tensegrity systems. �Although we made several assumptions, our model is an important step toward our goal of understanding the molecular basis of cell membrane mechanics,� said Sung. Sung, Skelton, and post-doctoral fellows Carlos Vera and Frederic Bossens combined mathematical modeling of a proto-filament as a tensegrity structure with a visualization technique that revealed how a single proto-filament moves in response to the pulling force of six spectrin fibers attached to it. Sung�s team was pleasantly surprised that its model also generated near-random yaw angles for the proto-filament during deformation of the red blood cell and no more than 18 degrees of pitch relative to the membrane in most cases. The modeling suggests that the more a red blood cell is mechanically deformed, the more likely its individual proto-filaments will rotate left and right like a baseball bat swung over home plate. �We think this model may explain why the deformations of red blood cells squeezing through narrow capillary openings are so important: the movement of proto-filaments may effectively enhance the diffusion of oxygen from red blood cells deep in tissues and organs where the exchange is most needed.�
The team is planning to broaden its analysis to include the effects of trans-membrane proteins that physically anchor the underlying protein network to the red blood cell membrane. The team also plans to enlarge its simulation to visualize more than one proto-filament at a time, and eventually model the simultaneously movement of all 33,000 proto-filaments in a cell. All rights reserved by www.rxpgnews.com |