
|
 |
Common brain cells may have stem-cell-like potential
Aug 17, 2006 - 4:02:00 PM, Reviewed by: Dr. Venkat Yelamanchili
|
|
"It was a long and difficult process, but we were able to induce what are basically support cells in the human brain to form beautiful new neurons in a dish"
|
By University of Florida,
University of Florida researchers have shown ordinary human brain cells may share the prized qualities of self-renewal and adaptability normally associated with stem cells.
Writing online in Development, scientists from UF's McKnight Brain Institute describe how they used mature human brain cells taken from epilepsy patients to generate new brain tissue in mice.
Furthermore, they can coax these pedestrian human cells to produce large amounts of new brain cells in culture, with one cell theoretically able to begin a cycle of cell division that does not stop until the cells number about 10 to the 16th power.
"We can theoretically take a single brain cell out of a human being and - with just this one cell - generate enough brain cells to replace every cell of the donor's brain and conceivably those of 50 million other people," said Dennis Steindler, Ph.D., executive director of UF's McKnight Brain Institute. "This is a completely new source of human brain cells that can potentially be used to fight Parkinson's disease, Alzheimer's disease, stroke and a host of other brain disorders. It would probably only take months to get enough material for a human transplant operation."
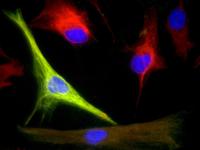 |
| UF McKnight Brain Institute researchers were able to purify and grow highly adaptable cells called adult human neural progenitors from mature human brain tissue. The green marker indicates a support brain cell called an astrocyte and the red marker is an indication of a stem cell, which is highly valued for its ability to transform into any cell type. Blue marks the cell nucleus. Credit: Noah Walton/UF McKnight Brain Institute |
The findings document for the first time the ability of common human brain cells to morph into different cell types, a previously unknown characteristic, and are the result of the research team's long-term investigations of adult human stem cells and rodent embryonic stem cells.
Last year, the researchers published details about how they used stem-like brain cells from rodents to duplicate neurogenesis - the process of generating new brain cells - in a dish. The latest findings go further, showing common human brain cells can generate different cell types in cell cultures. In addition, when researchers transplanted these human cells into mice, the cells effectively incorporated in a variety of brain regions.
The human cells were acquired from patients who had undergone surgical treatment for epilepsy and were extracted from support tissue within the gray matter, which is not known for harboring stem cells.
When the donor cells were subjected to a bath of growth agents within cell cultures, a type of cell emerged that behaves like something called a neural progenitor - a cell that is a bit further along in development than a stem cell but shares a stem cell's vaunted ability to divide and transform into different types of brain cells.
Even when the cells from the epilepsy patients were transplanted into mice, bypassing any growth enhancements, they were able to take cues from their surroundings and produce new neurons.
"It was a long and difficult process, but we were able to induce what are basically support cells in the human brain to form beautiful new neurons in a dish," said Noah Walton, a graduate student in the neuroscience department at the UF College of Medicine. "But what we really needed is for these support cells to turn into neurons in the brain, and we found we could get them to do it. Something in the environment in the rodent brain is sufficient to get these cells to become neurons."
Scientists speculate a small amount of existing progenitors may be emerging from the gray matter of the brain and multiplying in torrents, or perhaps the aging clock of the mature cells actually turns backward when the donor cells are in a new environment, returning them to past lives as progenitors or as stem cells.
"It's been shown that the same sorts of tissue from the mouse brain can give rise to rapidly dividing cells, but this shows it is true with human cells," said Ben Barres, M.D., Ph.D., a professor of neurobiology at the Stanford University School of Medicine who was not involved in the research. "That these cells were able to integrate into tissue in an animal model and actually survive - it was extremely important to show that. Now the question is what will these cells do in a human brain? Will they be able to survive for the long term and rebuild circuitry? This work is a first step toward that end."
In addition to using the cells in treatments to repair or replace damaged brain tissue, the ability to massively expand cell populations could prove useful in efforts to test the safety and efficacy of new drugs. It is also possible to genetically modify the cells to produce neurotrophins - substances that help brain tissue survive, researchers said. 
- Aug. 16 online issue of journal Development
www.ufl.edu
The research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Heart, Lung and Blood Institute of the National Institutes of Health. Steindler and co-senior author Bjorn Scheffler, M.D., a UF neuroscientist, are involved with RegenMed Inc., a biotechnology company that seeks to use stem cell technology to develop human therapeutics.
|
For any corrections of factual information, to contact the editors or to send
any medical news or health news press releases, use
feedback form
Top of Page
|
|
|
|