Gold Nanoparticle Molecular Ruler to Measure Smallest of Life’s Phenomena
Oct 12, 2006, 13:23, Reviewed by: Dr. Priya Saxena
|
|
“Our work promises to be a fast and convenient alternative for mapping DNA-protein interactions. We can measure precisely how a protein interacts with the information inscribed in the DNA and begins to regulate genetic information. We can also measure large protein-DNA interactions that span up to 17 nanometers in length, and, in theory, span as much as 70 nanometers in length.”
|
By Berkeley Lab,
Scientists from the U.S. Department Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California at Berkeley have developed a ruler made of gold nanoparticles and DNA that can measure the smallest of life’s phenomena, such as precisely where on a DNA strand a protein attaches itself.
The molecular ruler, detailed in the October premier issue of the journal Nature Nanotechnology, offers label-free and real-time measurement of a range of protein-DNA interactions at an extremely high resolution. As such, it promises to play a key role in the current push in biology to understand how genetic information flows from DNA to RNA to gene expression. Today, scientists involved in this research typically examine the final products of this chain of events by cataloging the expression levels of various genes and proteins.
The newly developed molecular ruler, however, can give scientists a much earlier glimpse into this process by measuring the initial protein-DNA binding interactions that unleash the flow of information which, in turn, sparks gene expression.
“We can use the ruler to look at this process much more upstream. We can measure the beginning stages of DNA-binding activities,” says Fanqing Frank Chen, a scientist in Berkeley Lab’s Life Sciences Division who was a member of the research team that, for the first time, used the molecular ruler to map protein-DNA interactions.
The existing techniques used to measure protein-DNA interactions involve labeling DNA and proteins with either radioactive or fluorescent compounds. But radioactive labels require tedious sample preparation and incur radiation-use restrictions, and fluorescent labels are short-lived and unable to measure complex protein-DNA interactions that measure more than 8 nanometers in length.
“Our work promises to be a fast and convenient alternative for mapping DNA-protein interactions. We can measure precisely how a protein interacts with the information inscribed in the DNA and begins to regulate genetic information,” says Chen. “We can also measure large protein-DNA interactions that span up to 17 nanometers in length, and, in theory, span as much as 70 nanometers in length.”
The molecular ruler was developed by a team of scientists that includes UC Berkeley Bioengineering Professor Luke Lee, UC Berkeley Ph.D. student Gang Liu, and Paul Alivisatos, Director of Berkeley Lab's Materials Sciences Division and an Associate Laboratory Director. It’s composed of gold nanoparticles that are coated with a substance that makes the nanoparticles soluble. Next, about 100 double-stranded DNA segments are tethered to the gold nanoparticle in a configuration that resembles a many-legged spider.
The ruler works because of plasmon resonance, which is the collection of electrons that resonate in a metallic particle, in this case the gold-DNA conjugate. Plasmon resonance changes as a particle changes, leading to differences in scattering wavelength. For example, if the gold particle’s spidery DNA strands, which are 54 base pairs long, are shortened for whatever reason, then the gold-DNA particle’s scattering wavelengths also shift — and this shift can be easily detected using spectroscopy. This method is so sensitive that scientists can use it to detect whether a DNA strand has been shortened by as little as one base pair in length, which opens the door for mapping the exact location of protein-DNA interactions.
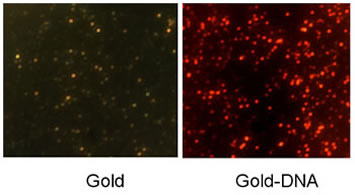 |
| These before-and-after images reveal how the gold nanoparticles change after DNA strands are added the nanoparticles. Chen and colleagues use these shifts in plasmon resonance to measure how proteins bind to DNA (Image: Berkeley Lab). |
Chen and colleagues put the ruler to the test by using it to conduct DNA footprinting, a process in which scientists identify where on a DNA strand a particular protein attaches itself. DNA footprinting is most commonly performed on proteins that are thought to play a significant functional role, such as in regulating gene expression.
To conduct this genetic sleuthing, they developed a customized gold-DNA conjugate. As usual, they attached to each gold nanoparticle roughly 100 DNA strands that are 54 base pairs long. But among these base pairs they inserted a sequence of six base pairs that are specially tailored to bind to a model protein, in this case EcoRI(Q111). In other words, at the same location on each strand, they encoded the perfect home for an EcoRI(Q111) protein. They introduced this protein to the specially prepared gold-DNA conjugates, and allowed the protein to bind to the DNA strands.
Next, to map exactly where the protein attaches to the DNA, they introduced an enzyme called an exonuclease. This enzyme clamps onto the free end of the DNA strands, and chomps down each strand, removing base pair after base pair, until it’s blocked by the recently attached EcoRI(Q111) protein. It’s like someone slurping down a spaghetti noodle, only to be stopped cold by a fly sitting on the noodle.
In this way, the gold particles’s DNA strands are shortened, with their newly sheared free ends marking the location of the protein. And this, in turn, allows the research team to zero in on the DNA’s protein binding site. They already know the plasmonic scattering signature of the gold-DNA particle with all of its 54 base pairs. Now, they can then measure the plasmonic signature of the gold-DNA particle after its DNA has been trimmed. The difference between the two spectra correlates to the number of base pairs eliminated by the exonuclease.
“The plasmon resonance wavelengths decrease by a certain number of nanometers, which translates to a certain number of DNA base pairs removed,” says Chen.
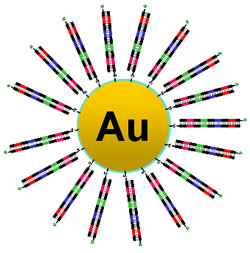 |
| By attaching DNA strands to gold nanoparticles, Berkeley Lab and UC Berkeley scientists have developed a ruler capable of measuring protein-DNA interactions (Image: Berkeley Lab). |
This allows Chen and colleagues to measure how far the exonuclease travels down the DNA strand, which enables them to determine precisely where the protein binding site is located. The result is a quick and relatively cheap glimpse into the earliest stages of genetic activity.
“We are monitoring the actual mechanism that causes genetic information to begin to flow, such as gene regulation, not the expression levels of genes and proteins, which are endpoint measurements” adds Chen.
In addition to DNA footprinting, the molecular ruler can be used to monitor any enzyme that causes length changes in DNA, such as nucleases that cleave DNA strands in two. And the molecular ruler’s ability to measure changes in a single nanoparticle without the need for radioactive or fluorescent labeling makes it possible to perform high-throughput screening in a high-density microarray or a microfluidic device. 
- Published in October premier issue of the journal Nature Nanotechnology. The paper is entitled: A nanoplasmonic molecular ruler for measuring nuclease activity and DNA footprinting.
www.lbl.gov
This research was funded by the Defense Advanced Research Projects Agency, the National Institutes of Health, the University of California at San Francisco’s Prostate Cancer Development Research Program, Intel Corporation, and the Korea Ministry of Science and Technology’s “21st Century Frontier R&D Program.”
|
For any corrections of factual information, to contact the editors or to send
any medical news or health news press releases, use
feedback form
Top of Page
|